Dry Hydrogen Peroxide (DHP®) Expands through Healthcare Facilities Nationwide, Providing Continuous Microbial Reduction
CLICK HERE to download the white paper
Introduction
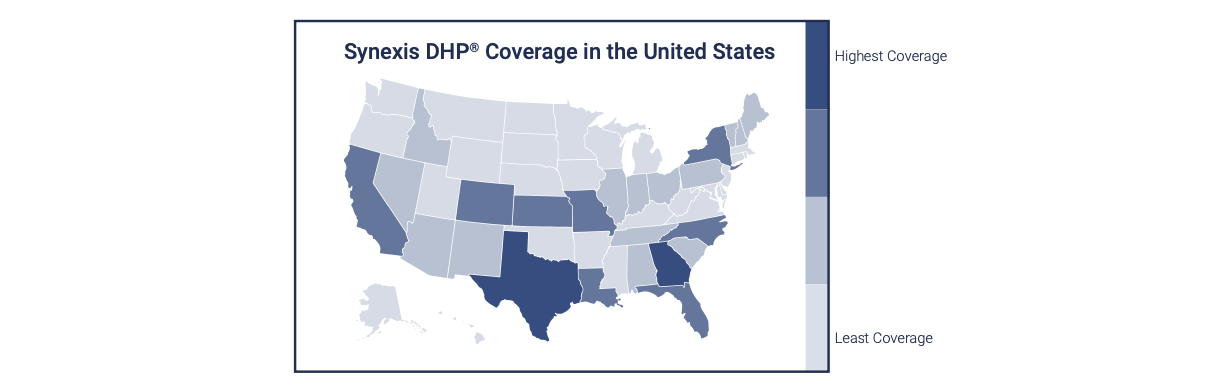
Dry Hydrogen Peroxide (DHP) is a true gas that is used for microbial reduction in occupied healthcare settings.1 On a facility level, DHP flows throughout a treated space, eliminating microbes from floor to ceiling and corner to corner. On a national level, DHP is flowing throughout some of the country’s largest for-profit and not-for-profit health systems, coast to coast and region to region. The reason for the rapid rise in DHP deployment is not a mystery.
There are well-established challenges in environmental infection prevention and control (IPC). Environmental surfaces in healthcare settings are often sub-optimally cleaned and disinfected, leading to an increased risk of infection transmission.2 Many microbes left behind by inadequate cleaning and disinfection can survive in the environment for days to weeks, if not longer.2 Even when the most comprehensive terminal cleaning and disinfection protocols are perfectly executed, recontamination is inevitable when a person—healthcare worker, patient, or visitor—enters the space.2-3 If you add to these facts the recent development of a critical shortage in healthcare workers, including environmental service technicians,4 an opportunity for process improvement within the field of infection prevention and control becomes very apparent. Enter DHP.
DHP addresses these obstacles head-on. As a true gas, DHP flows wherever the air flows, including into remote recesses (e.g., drawers, cabinets, etc.) or hard-to-reach surfaces (e.g., window sills, high shelves, etc.). In other words, DHP can’t “miss” a spot on a surface, let alone an entire surface. Instead, it attacks microbes in the air and on surfaces throughout the entire treated space. DHP is created from the air in the room and operates continuously, releasing DHP around the clock and allowing it to attack microbes 24/7/365. DHP helps to reduce the steady state of bioburden (i.e., bacteria, viruses, and fungi, including molds), augmenting manual cleaning and disinfection efforts.
This continuous operation in occupied settings also allows DHP to address contamination as it occurs, instead of just addressing it on an intermittent, single point-in-time basis.
Further, the technology that creates DHP is fully automated and does not require staff deployment or oversight, room preparation, or room vacancy. In other words, it does not require additional staffing or more of your existing staff.
What is DHP?
Hydrogen peroxide is a time-tested disinfectant. As stated by the Centers for Disease Control and Prevention (CDC), it is “bactericidal, virucidal, sporicidal, and fungicidal.”5 However, all hydrogen peroxide is not the same; it comes in many forms. Dry Hydrogen Peroxide, as the name implies, is not stabilized by water, unlike all pre-mixed solutions used for manual cleaning or use in hydrogen peroxide vapor or mist technologies.1 In contrast, DHP is hydrogen peroxide in a true gas form and at a concentration safe for occupied spaces.
Synexis technology utilizes ambient oxygen and humidity (i.e., naturally found in the air in the room or space) to create DHP. The humidity and oxygen are pulled through the device where a molecular change involving a UV-A bulb (not to be confused with UV-C bulbs) and a proprietary catalyst occurs. This photocatalytic process re-orients the oxygen and humidity molecules, dispelling DHP, the gaseous phase of Hydrogen Peroxide, into the treated space.1 DHP does not contain moisture and does not accumulate in the environment.
How Does DHP Work?
When DHP is released from the device, it travels with the air inside the treated space, flowing from corner to corner, floor to ceiling, and inside drawers, cabinets, and other recessed spaces. No object can be “in the way” because, like air, DHP simply flows around it. In other words, there are no line of sight issues or need to open drawers and cabinets as with UV-C decontamination.
The real action occurs when DHP encounters a microbe. Microbes need moisture to survive and contain external sites that attract water. Hydrogen peroxide is structured similarly to water, so it is attracted to these same sites. Once DHP encounters a microbe, whether a bacteria, virus, or fungus, it then initiates a series of reactions that cause the microbe to rupture. At the same time, DHP is broken back down into water and oxygen.
What is the Difference between DHP and Hydrogen Peroxide Vapor or Mists?
Put simply, water is the fundamental difference between DHP and hydrogen peroxide vapor (also called vaporized hydrogen peroxide/VHP) or hydrogen peroxide mist (also called aerosolized hydrogen peroxide/aHP).1 Although Synexis technology uses humidity to create DHP, DHP itself is non-aqueous—a true gas. VHP and aHP systems require aqueous solutions of hydrogen peroxide that are added to their respective devices before deployment, and the vapor or mists generated, as the names imply, contain water.
Remember how hydrogen peroxide is attracted to vulnerable sites on a microbe because it is so structurally similar to water? Imagine then what happens when hydrogen peroxide is mixed with water molecules. It must compete with them for those vulnerable sites on a microbe. That means that to eliminate microbes in the presence of water effectively, the number of hydrogen peroxide molecules (i.e., the concentration of hydrogen peroxide) needs to be higher.
This is why VHP and aHP use much higher concentrations of hydrogen peroxide than DHP. VHP technologies typically use a solution with 30-35% hydrogen peroxide mixed with water.2 Aerosolized hydrogen peroxide systems use a solution with 5-7% hydrogen peroxide combined with silver cations (<50 parts per million [ppm] Ag+).2 The concentrations of hydrogen peroxide achieved by both VHP and aHP exceed the safety limits established by the Occupational Safety and Health Administration (1 ppm or 1,000 parts per billion as an 8-hour time-weighted average).6 This explains why both technologies can only be used in unoccupied spaces that have been sealed off to prevent leakage of harmful concentrations of the vapor or mist.2
By contrast, DHP, as a true gas without moisture, does not have to compete with water molecules to engage with microbes so that it can be effective at dramatically lower concentrations of hydrogen peroxide. In fact, at the high end, the concentration of hydrogen peroxide achieved by DHP is less than 2 parts per billion, far below the 1 part per million safety threshold. This is why DHP can be safely used around the clock in occupied spaces.
This also means that DHP’s effect is achieved at a slower, steadier pace. VHP and aHP, given their higher concentrations of hydrogen peroxide, have a more immediate impact in reducing microbes. However, they can only be used in unoccupied spaces and they can’t be used continuously, allowing for rapid recontamination. VHP and aHP also require staff oversight for vacating the room, preparing it for treatment (sealing doors, windows, and vents), deploying the device, and subsequently unsealing the room.7
Table 1: Key Operational Differences between Commercially Available Forms of Hydrogen Peroxide for Whole Room Disinfection7-8
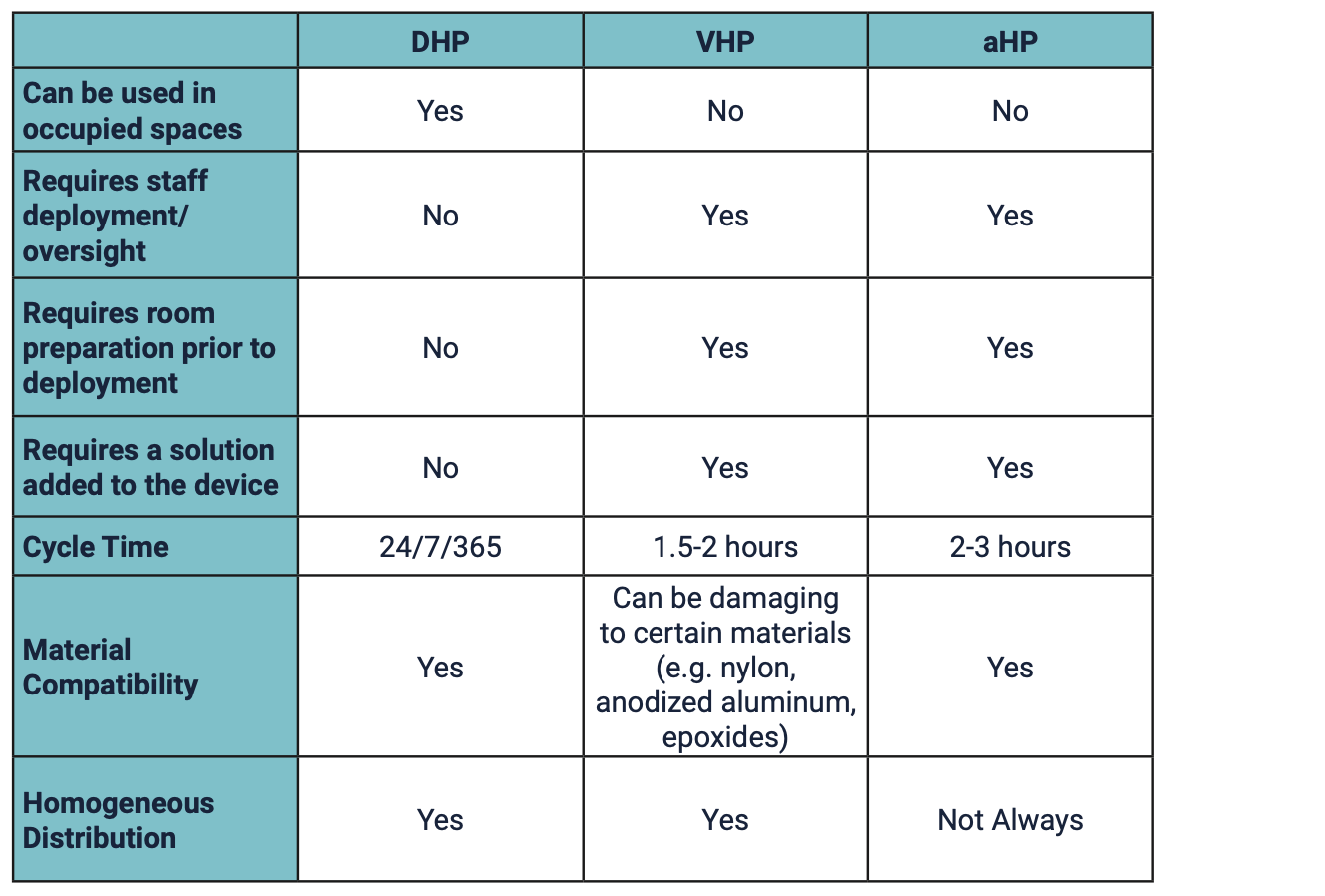
Understanding that hydrogen peroxide comes in different forms is important because of the different use parameters and safety profiles1 but the terminology can be confusing. For example, some aHP technologies are identified as producing a “dry hydrogen peroxide mist” when, in fact, the mist produced contains water.7,9 Other products claim to use continuous, permeable, ultra-low-level hydrogen peroxide. However, there is only one Dry Hydrogen Peroxide. Synexis is the developer and sole manufacturer of DHP.
DHP and Your Facility
Synexis technology is available in three devices, depending on a facility’s needs. The Blade fits in line with existing HVAC system ductwork without affecting the system’s operation. The portable Sphere plugs into a standard 120VAC/220VAC electrical outlet and can be placed or mounted anywhere and moved as needed. Like the Sphere, the Sentry XL plugs into a standard 120VAC outlet and is portable but designed for use in larger spaces, measuring up to 3,000 square feet.
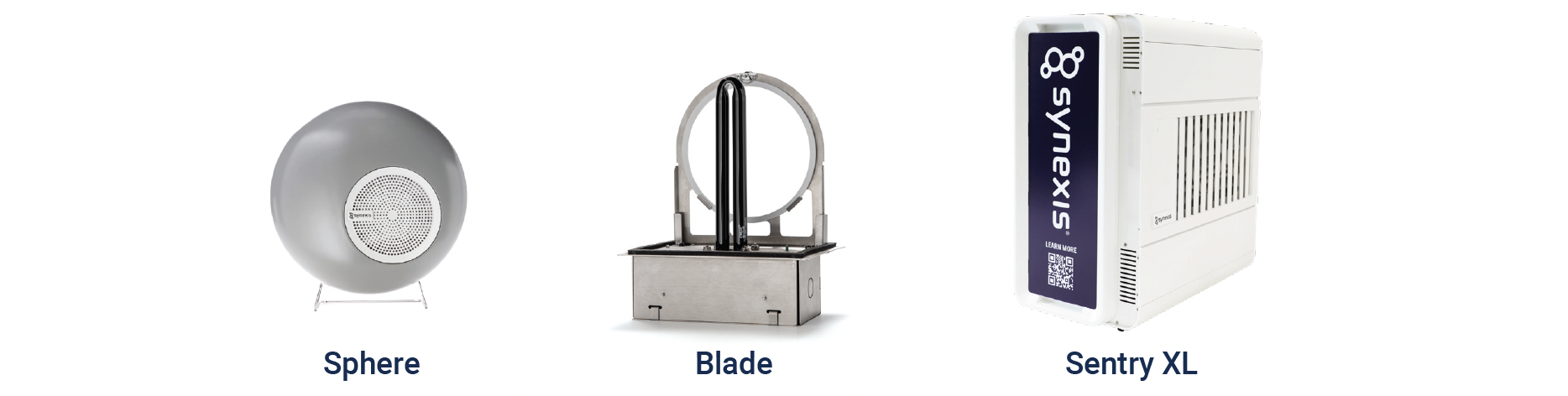
The number of devices required is based on the area of intended use, including (among other factors) size, layout, and HVAC parameters. A Field Operations team reviews these factors to develop a detailed deployment plan. Once installed in a facility, the devices are fully automated and run continuously. And the only maintenance needs are twice-a-year changes of the catalyst (i.e., “sail”).
The bottom line is that Synexis technology does not add to a Facility Manager, EVS Director, or EVS technician’s workload. DHP operates independently, augmenting a facility’s standard manual cleaning and disinfection protocol.
Efficacy / Evidence
Technology may seem ideal in theory, but in healthcare, the real test comes from peer-reviewed evidence. Has it been tested in real-world clinical environments? Have the results been statistically significant? Did the study design control for other potentially confounding variables, such as other technologies or infection prevention initiatives? Has research shown an impact on reducing bioburden in occupied settings? Has the research shown an impact on reducing healthcare-associated infections?
For DHP, the answer to these questions is a resounding “yes.” DHP has been evaluated in eleven peer-reviewed studies conducted in a variety of settings, including the intensive care unit (ICU) of a pediatric oncology hospital, an adult burn unit in a tertiary care hospital, multiple units within a large acute care hospital, a freestanding Emergency Department (ED), and a long-term care facility, among others.10-20 The studies have incorporated various design features, including untreated control units and before/after design (the most common design for no-touch technology evaluations)1, assessment of total microbial reduction or specific pathogen reduction (e.g., Candida auris), and single unit or multicenter setting.
Table 2 outlines the peer-reviewed evidence demonstrating DHP’s efficacy in reducing environmental bioburden and/or healthcare-associated infections. While no existing technology can unilaterally prevent HAIs or eliminate the need for manual cleaning, Synexis technology has proven efficacy in comprehensively reducing bioburden through continuous operation in actively occupied settings.
Author, Year |
Journal |
Setting |
Selected Results |
|
Sutton et al, 2023 |
Burn ICU, Burn Stepdown Unit |
↓ C. auris bioburden |
|
|
Wright et al, 2023 |
2 Tertiary Care Hospitals, 1 Free-Standing ER |
↓ Air and surface bioburden |
|
|
Sanguinet et al, 2023 |
Adult Burn ICU, Children Cardiac Unit |
↓ C. auris bioburden |
|
|
Cole, 2023 |
Neurobehavioral Unit of LTCF |
↓ Surface bioburden |
|
|
Melgar et al, 2023 |
Pediatric Oncology ICU |
↓ HAIs |
|
|
Huang et al, 2021 |
50m2 BSL 3 Lab |
↓ SARS-CoV-2 bioburden |
|
|
Sanguinet et al, 2021 |
Tertiary Care Hospital |
↓ Bioburden on privacy curtains |
|
|
Sanguinet et al, 2021 |
Tertiary Care Hospital |
↓ Air and Surface Bioburden |
|
|
Ramirez et al, 2021 |
Pediatric Oncology ICU |
↓ Surface Bioburden |
|
|
Herman et al, 2015 |
Cardiovascular Telemetry Unit |
↓ Surface Bioburden |
|
|
Kaiser et al, 2020 |
Clean Room |
Achieved |
Case Study—Sunrise Hospital, Las Vegas, NV
Jennifer Sanguinet, DrPh, FAPIC, CIC, MBA-HCM, BSIS, Director of Infection Prevention for Sunrise Hospital and Medical Center, Las Vegas’s largest acute care facility, is always on the lookout for opportunities for process improvement that offer solutions without creating new challenges. Like hospitals nationwide, the facility has faced staffing shortages in recent years, complicating efforts to reduce the risk of environmental infection transmission through effective cleaning and disinfection.
In 2019, she embarked on a 12-month study to evaluate DHP as an environmental strategy that would augment her facility’s manual cleaning and disinfection protocol without further taxing her EVS staff. DHP was initially deployed in five units—the pediatric intensive care unit, pediatric ED, adult oncology unit, adult ICU/cardiovascular trauma unit, and adult trauma surgical ICU. After finding that DHP was effective at reducing air and surface bioburden throughout the treated units (a reduction of 96.5% for surface bioburden, including 99.5% for privacy curtains alone) and the added burden of novel organisms (C. auris), she expanded the deployment of DHP throughout the entire facility.17-18
This has proven to be especially valuable considering the dramatic rise in C. auris cases throughout Nevada, impacting over 30 facilities and putting the state at the top of the list regarding case numbers.21-22 In a study published last year, Sanguinet found that DHP successfully reduced C. auris environmental contamination within and around the rooms of affected patients.12
Describing her experience with the automated, continuous technology, Sanguinet said, “It is an IP’s dream to have an environmental technology that is effective on such a broad scope and scale. But it is a true game-changer when that technology can operate independently, around the clock, in occupied spaces.
We have significantly reduced contamination of clinically relevant pathogens throughout our facility with DHP. We’ve also experienced other ancillary benefits, including reduced noxious odors. For example, our medivac helicopter landing pad is directly above a patient care unit, and staff routinely complained of fumes emanating from the helicopter. Since deploying DHP, those odors have disappeared, and we’ve also seen dramatic reductions in odors from other volatile organic compounds, including those from human waste and secretions.”
Why DHP?
As a fully automated, continuously operational technology, DHP is a silent, behind-the-scenes technology with proven efficacy across the continuum of care. While efficacy is arguably the most critical factor when choosing a “no-touch” decontamination technology, practical considerations don’t rank far behind. In one review, experts conclude that determining which technology best suits a facility depends heavily on these considerations, including “duration of disinfection, the preparations prior to disinfection, the purchase and operating costs, and the user-friendliness of a device.”7
DHP is unique in that, aside from initial purchase cost and electricity for operation, there are essentially no operational considerations to account for. DHP integrates seamlessly into a facility’s operation, safeguarding the environment of care through continuous microbial reduction. Let this novel form of a well-established disinfectant enhance your environmental infection prevention strategy without requiring more of your environmental team.

References
1. Lee, Chris, and John R. Henneman. ‘Dry Hydrogen Peroxide for Viral Inactivation’. Disinfection of Viruses, IntechOpen, 18 May 2022. Crossref, doi:10.5772/intechopen.100451.
2. Weber DJ, Rutala WA, Anderson DJ, Sickbert-Bennett EE. .. No touch methods for health care room disinfection: Focus on clinical trials. Am J Infect Control. 2023 Nov;51(11S):A134-A143. doi: 10.1016/j.ajic.2023.04.003.
3. Hardy KJ, Gossain S, Henderson N, Drugan C, Oppenheim BA, Gao F, Hawkey PM. Rapid recontamination with MRSA of the environment of an intensive care unit after decontamination with hydrogen peroxide vapour. J Hosp Infect. 2007 Aug;66(4):360-8. doi: 10.1016/j.jhin.2007.05.009.
4. https://professional.contecinc.com/blog/innovative-approach-to-the-evs-worker-crisis
5. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/disinfection-methods/chemical.html
6. https://www.osha.gov/chemicaldata/630
7. van der Starre, C.M., Cremers-Pijpers, S.A.J., van Rossum, C. et al. The in situ efficacy of whole room disinfection devices: a literature review with practical recommendations for implementation. Antimicrob Resist Infect Control 11, 149 (2022). https://doi.org/10.1186/s13756-022-01183-y
9. Amodio E, Kuster SP, Garzoni C, Zinkernagel AS, Sax H, Wolfensberger A. Disinfecting noncritical medical equipment-Effectiveness of hydrogen peroxide dry mist as an adjunctive method. Am J Infect Control. 2020 Aug;48(8):897-902. doi: 10.1016/j.ajic.2020.05.016.
10. Kaiser C, Roe AM. Infection Preventionists and Pharmacists Share Responsibility for Ensuring Patient Safety. Pharmacy Times Nov 2020.
11. Sutton JA, Dotson N, Nagy J, Moody J, Sands KE, 1418. The Effect of Dry Hydrogen Peroxide on Environmental Contamination with Candida auris in an Adult Burn Unit, Open Forum Infectious Diseases, Volume 10, Issue Supplement_2, December 2023, ofad500.1255, https://doi.org/10.1093/ofid/ofad500.1255
12. Wright D, Christie J, Lawrence J, Vaughn KL, Walsh TF. Effectiveness of dry hydrogen peroxide in reducing air and surface bioburden in a multicenter clinical setting. Infect Control Hosp Epidemiol. 2023 Nov 29:1-8. doi: 10.1017/ice.2023.153.
13. Sanguinet J, Marshall G, Moody J, Sands K. Effect of dry hydrogen peroxide on Candida auris environmental contamination. Antimicrob Steward Healthc Epidemiol. 2023 Sep 29;3(Suppl 2):s67–8. doi: 10.1017/ash.2023.316.
14. Cole M. Impact of dry hydrogen peroxide on environmental bioburden reduction in a long-term care facility. Am J Infect Control. 2023 Dec;51(12):1344-1349. doi: 10.1016/j.ajic.2023.06.004
15. Melgar M, Ramirez M, Chang A, Antillon F. Impact of dry hydrogen peroxide on hospital-acquired infection at a pediatric oncology hospital. Am J Infect Control. 2022 Aug;50(8):909-915. doi: 10.1016/j.ajic.2021.12.010.
16. Huang YS, Bilyeu AN, Hsu WW, Hettenbach SM, Willix JL, Stewart SC, Higgs S, Vanlandingham DL. Treatment with dry hydrogen peroxide accelerates the decay of severe acute syndrome coronavirus-2 on non-porous hard surfaces. Am J Infect Control. 2021 Oct;49(10):1252-1255. doi: 10.1016/j.ajic.2021.07.006.
17. Sanguinet J, Lee C. An effective and automated approach for reducing infection risk from contaminated privacy curtains. Am J Infect Control. 2021 Oct;49(10):1337-1338. doi: 10.1016/j.ajic.2021.06.004.
18. Sanguinet J, Edmiston C. Evaluation of dry hydrogen peroxide in reducing microbial bioburden in a healthcare facility. Am J Infect Control. 2021 Aug;49(8):985-990. doi: 10.1016/j.ajic.2021.03.004.
19. Ramirez M, Matheu L, Gomez M, Chang A, Ferrolino J, Mack R, Antillon-Klussmann F, Melgar M. Effectiveness of dry hydrogen peroxide on reducing environmental microbial bioburden risk in a pediatric oncology intensive care unit. Am J Infect Control. 2021 May;49(5):608-613. doi: 10.1016/j.ajic.2020.08.026
20. Herman, CK, Hess, J, Cerra, C. Dilute hydrogen peroxide technology for reduction of microbial colonization in the hospital setting. Am J Infect Control 2015;43(6):S25–S26
21. https://www.unlv.edu/news/release/unlv-snwa-study-makes-case-candida-auris-wastewater-surveillance
22. https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html
