From time to time, we will turn the Synexis blog over to our research and development team. Sometimes, they’ll be quick looks into the data, while other times will be longer series. This is a longer series. It is Part One of a four-part series of blogs from the Advanced Technology Group to discuss the chemistry of Dry Hydrogen Peroxide (DHP®). To look ahead, this series will consist of the following topics:
- Part 1: What is DHP?
- Part 2: Why is DHP more reactive than aqueous H202?
- Part 3: How do we know we produce DHP vs. wet (aqueous) H202?
- Part 4: How do we measure DHP?
From a scientific point of view, “DHP is the non-fully hydrated form of hydrogen peroxide.” However, for those not steeped in chemistry lingo – this can be better described with a series of images.
Dry Hydrogen Peroxide Chemical Formula
First, the chemical formula of DHP is H2O2, with the bonding structure in Figure 1 below. While it is easier to visualize H2O2 using ball and stick figures (Figures 1A and 1B), space-filled models show how much space each atom occupies. This becomes an important discussion point when discussing why DHP is more reactive compared to hydrated versions of H2O2.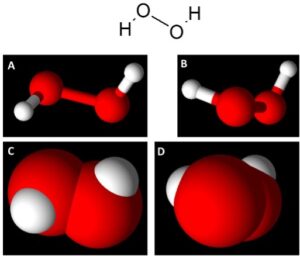
Figure 1: Representation of H2O2. Upper Image: chemical structure of H2O2 showing the bonding interactions between hydrogen and oxygen. (A&B): ball & stick figures of H2O2 from different angles showing the bonding of hydrogen (white atoms) to oxygen (red atoms). (C&D): space filled models of H2O2 from different angles.
Hydrogen Bonds
Because hydrogen is bound to a highly electronegative element (oxygen), it can form weak bonds called hydrogen bonds1. When hydrogen peroxide is fully hydrated (as shown in Figure 2), two interactions can occur:
- The hydrogens on H2O2 can interact with oxygen in water to stabilize the H2O2.
- The oxygens on H2O2 can interact with the hydrogens in water to stabilize the H2O2.
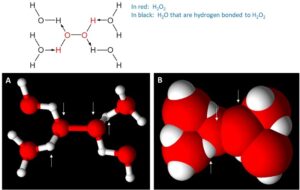
Figure 2: Images representing fully hydrated H2O2. Upper Image: Chemical structure showing the H2O2 molecules (in red) forming hydrogen bonds with four different H2O molecules (black). (A): ball and stick representation of the same structure with white arrows pointing out the atoms (red being oxygen atoms, and white being hydrogen atoms) of H2O2. (B): space filled model representation of H2O2 that has been fully hydrated.
Examination of Figure 2b shows that the hydrogen atoms are very difficult to ‘see’ in H2O2. These water molecules forming hydrogen bonds to the H2O2 molecule are also stabilizing the hydrogen peroxide. This stabilization arises from the fact that they cause steric hindrance2. This steric hindrance makes it harder for the H2O2 to interact with other molecules and/or organisms, thereby reducing its efficacy in reducing VOCs (volatile organic compounds) and killing microorganisms.
It should be pointed out that this is the simplest fully hydrated version of hydrogen peroxide. In reality, more water molecules can form hydrogen bonds with the structure depicted in Figure 2, and as such, the reactivity of the H2O2 is reduced even further. A slightly extended structure of H2O2 that is more fully hydrated is shown in Figure 3.
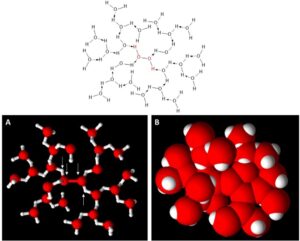
Figure 3: Images representing an extended hydrogen-bonded stabilized H2O2 molecule. Upper Image: chemical structure of H2O2 (in red) with an extended hydrogen-bonded water network (in black). (A): ball and stick representation of the same chemical structure with white arrows showing the location of the H2O2 atoms. (B): space-filled model representation with the red spheres representing oxygen and the white spheres representing hydrogen.
Examination of Figure 3B shows that extending the hydration sphere hinders physical access to the H2O2 molecule. This further illustrates that DHP should be more reactive than the hydrated version of H2O2. This is due to the lack of steric hindrance associated with the water molecules that hydrogen bond with the H2O2 (compare Figure 1 – DHP vs. Figure 3 – hydrated H2O2).
DHP vs. Hydrogen Peroxide
Message: DHP is the non-fully hydrated version of hydrogen peroxide.
Message: DHP is more reactive than aqueous versions of H2O2.
Why: One of the reasons is increased steric hindrance when H2O2 forms hydrogen bonds with H2O molecules.
1A hydrogen bond, as defined by Oxford Languages Dictionary, is: “A weak bond between two molecules resulting from an electrostatic attraction between a proton (hydrogen atom) in one molecule and an electronegative atom in the other.” Electronegative elements that exhibit strong tendencies for hydrogen bonding include: O (oxygen), S (sulfur), N (nitrogen), P (phosphorus), Cl and (chlorine).
2Steric hindrance, as defined by Dictionary.com, is: “The prevention of retardation of inter- or intramolecular (reactivity) interactions as a result of the spatial structure of a molecule.”









